While genetics has long been viewed as a major determinant of diabetes, scientists are now discovering that genes alone don’t tell the full story. The emerging field of epigenetics, the study of how behaviors and environments can alter gene expression without changing the DNA sequence, offers a deeper, more dynamic understanding of diabetes risk. Industry professionals like Joe Kiani, founder of Masimo, understand the importance of research that explores these deeper molecular influences, particularly when they lead to more personalized approaches to disease prevention.
This shift in focus is helping scientists identify how diet, stress, physical activity and other day-to-day experiences can affect how genes are expressed, including those linked to glucose regulation and insulin function. As a result, epigenetics is not only changing how risk is defined, but also how it might be reduced. This brings us one step closer to prevention strategies tailored to the individual.
What Is Epigenetics and Why Does It Matter?
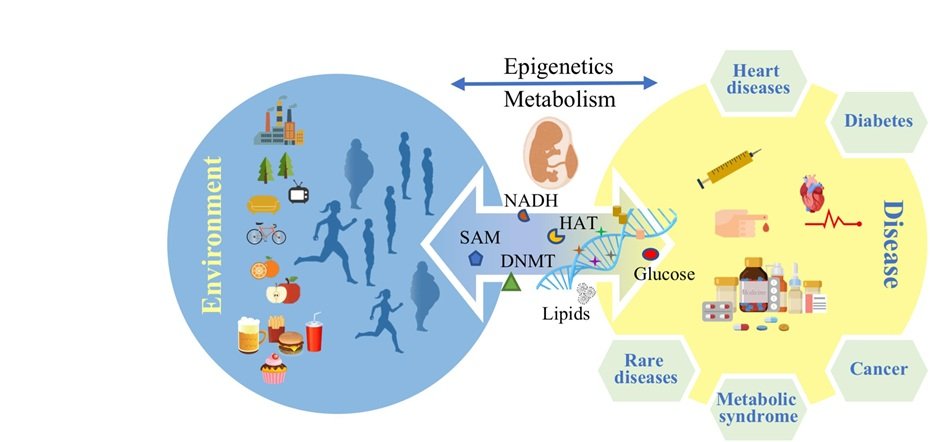
Epigenetics focuses on how external factors like diet, stress, exercise, pollution or even trauma can influence the way genes are turned on or off. These changes, known as epigenetic modifications, can affect how the body processes glucose, regulates insulin and manages inflammation, all of which play a key role in the development of Type 2 diabetes.
The most studied epigenetic mechanisms include DNA methylation, histone modification and non-coding RNA expression. These changes can be triggered by lifestyle and environmental exposures and may even be passed down to future generations. This means your risk of diabetes may be influenced not just by your habits but also by those of your parents or grandparents.
Linking Epigenetics to Diabetes Development
Researchers have found that people with diabetes often display unique epigenetic markers that differ from those without the disease. For example, specific methylation patterns have been observed in genes related to insulin secretion and glucose metabolism. These markers may not just reflect disease presence; they could also indicate predisposition, offering early warning signs long before clinical symptoms appear.
This insight opens the door to intervention. If a person is found to carry high-risk epigenetic signatures, they might benefit from lifestyle changes or monitoring well before blood glucose levels rise. That shift from reaction to prediction is a key advantage of incorporating epigenetics into diabetes research.
Environmental Factors and Intergenerational Impact
One of the most compelling aspects of epigenetics is its sensitivity to environmental influences. Diet, for instance, has been shown to directly alter epigenetic markers. High-fat, low-fiber diets may contribute to changes that impair insulin sensitivity, while diets rich in fruits, vegetables and whole grains can support more favorable gene expression.
The effects don’t stop with the individual. Studies in both humans and animals suggest that epigenetic changes can be transmitted across generations. A mother’s diet during pregnancy, for example, may influence her child’s metabolic profile, increasing or decreasing their risk of developing diabetes later in life. This makes epigenetic research especially valuable for shaping early-life interventions.
Epigenetics and the Promise of Personalized Prevention
Unlike static genetic information, epigenetic modifications are malleable. They can change over time based on lifestyle, which means they offer a powerful opportunity for prevention. With advances in testing, clinicians may one day be able to evaluate an individual’s epigenetic risk for diabetes and create a tailored plan that includes dietary guidance, physical activity and stress reduction strategies.
This marks a departure from traditional risk models, which often rely heavily on family history or the body mass index. Epigenetic data adds another layer, one that reflects real-time influences and biological responses. The result is a more holistic view of risk that empowers patients with information they can act on.
The push toward more personalized, actionable insight mirrors other breakthroughs aimed at simplifying chronic disease management. One example is the development of tools that prioritize real-time data and ease of use in daily care routines. As Joe Kiani said, “Epigenetics offers a new lens through which we can understand diabetes risk and prevention showing us how lifestyle, environment, and even early life experiences can shape our health in ways we’re only beginning to explore.” That focus on usability and long-term impact aligns with the goals of epigenetic research, which seeks to translate complex biological signals into guidance people can understand and act on.
Epigenetic Biomarkers and Clinical Application
Researchers are working to identify reliable epigenetic biomarkers that could be used in routine screenings. These biomarkers might one day be included in blood tests or other assessments to determine a person’s risk level and guide preventative care. Some early studies suggest that specific methylation patterns can serve as indicators of metabolic dysfunction even before glucose levels shift.
While this work is still in its early stages, the potential is enormous. Incorporating epigenetic screening into public health initiatives could allow for more targeted interventions and earlier detection. It also opens new possibilities for drug development by identifying novel targets involved in glucose regulation and insulin signaling.
Ethical Considerations in Epigenetic Research
As with any health technology, the use of epigenetic data raises ethical questions. Who owns the data? How should it be stored or shared? Could it affect insurance coverage or employment decisions? These questions must be carefully addressed through clear regulation, informed consent processes and public dialogue.
Because epigenetics touches on deeply personal factors like socioeconomic status and maternal health, it’s essential that the research isn’t used to assign blame. Instead, it should be a tool to promote fairness, helping everyone access the preventive care and education they deserve.
Challenges and Opportunities Ahead
Despite its promise, epigenetic research faces several challenges. Epigenetic modifications can be tissue-specific, meaning that markers found in blood may not reflect changes happening in muscle or pancreas tissue. Large, diverse datasets are needed to validate findings across populations.
Still, momentum is growing. Collaborative efforts between academic institutions, health systems and technology companies are expanding the reach and accuracy of epigenetic science. As the research matures, the vision of translating it into meaningful, actionable healthcare will move closer to reality.
Redefining Risk Through Epigenetics
Epigenetics is reshaping our understanding of what influences diabetes risk not just at the genetic level but across generations, environments and lifestyles. Its ability to reveal modifiable pathways offers hope for a new kind of prevention, one that begins earlier, adjusts over time and considers the individual as more than just a collection of genes.
As science continues to push the boundaries of what’s possible, the future of diabetes prevention may soon be rooted in molecular precision. Epigenetics doesn’t just tell us who’s at risk; it tells us where we can intervene, offering new tools to delay or even prevent the onset of one of the world’s most persistent chronic diseases.













Leave a Reply